- Home
- >
- Curriculum
- >
- Subjects
- >
- Physics
- >
- Physics Fun Fact
-
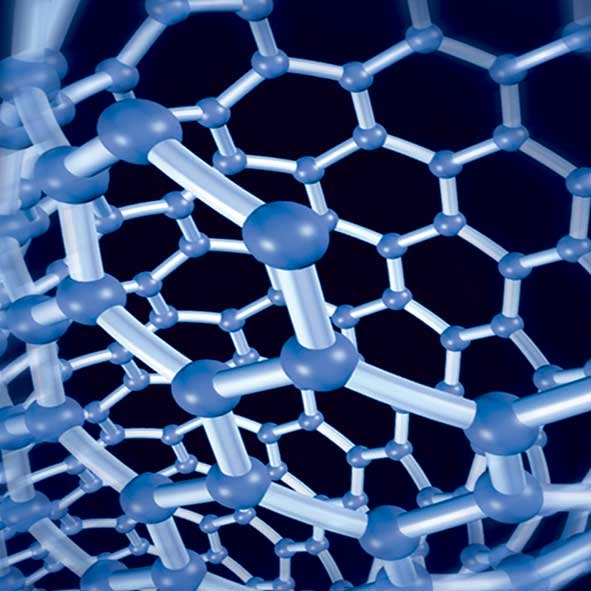
Nano (short for nanometer, symbol nm) is actually an abbreviation for nanometer, which is one of the units of length. 1 nanometer (1x10^-9 m) is equal to one billionth of a meter, and it is a very small length. The diameter of a human hair is approximately 60,000 to 80,000 nanometers. And the length of one nanometer is approximately equal to the length of ten hydrogen atoms lined up in a row. If the size of an object is less than 100 nanometers, then it can be called a nanomaterial. You may wonder why the boundary is set at 100 nanometers instead of 1000 or 10 nanometers.
The reason is that when the size of a material is less than 100 nanometers, its physical and chemical properties undergo significant changes. For example, the surface effect of nanomaterials plays an important role. It can be seen that as the cubes are divided into smaller cubes, the ratio of total surface area to volume increases. So if we have a pile of gold grains with a total weight of one gram, and each grain is smaller than 100 nanometers, then their surface area becomes very important.
However, we know that the surface of an object (relative to its interior) is very unstable and most active in chemical reactions. Therefore, increasing the surface area greatly accelerates the chemical reactions, surface absorption capacity, and catalytic ability of the substance. Moreover, the thermal, mechanical, and optical properties of nanomaterials are significantly different from those of larger materials. For example, the melting point of a piece of gold is approximately 1000 o C, but if it is a gold grain with a size of only 2 nanometers, its melting point decreases to 330 o C. Furthermore, the light absorption capacity of nanomaterials is also significantly enhanced. In addition, when the size of an object is less than 100 nanometers, a new phenomenon (which cannot be explained by classical physics) begins to emerge. It is because of these changes at the nanoscale that a new class of materials is created.
Can we manufacture granular nanoscale materials? Can we use a building block approach to assemble molecules or atoms into a new class of materials? The answer is yes. This nanotechnology indeed exists, and it is thanks to this advanced technology that nanoscience is feasible. The ability to move atoms one by one has always been a dream of scientists.
Nobel laureate Richard Feynman said in 1959: "If we could observe the technology of atoms with complete development, this technology could greatly help solve chemical and biological problems, and I think the development of this technology is inevitable." In fact, now we can manipulate atoms using a scanning tunneling microscope (STM). The scanning tunneling microscope is an instrument that can magnify the substance on the surface of an object to the atomic level, and it can be used to move atoms and create various interesting atomic structures (see Figure 1: Xenon on Nickel). The invention of the scanning tunneling microscope is truly revolutionary and essential for studying nanotechnology, and the inventor of this instrument also became the recipient of the 1986 Nobel Prize in Physics.
In addition to a deep interest in basic science, another driving force behind our research in nanoscience is, of course, its applications. Many people now believe that nanoscience can bring about a new industrial revolution. In brief, nanotechnology has been applied in four areas: (1) nanoelectronics: electronic instruments with new functions, which have the advantages of high speed and low energy consumption; (2) nanomaterials science: new materials with new properties - ideal materials for manufacturing nanoscale instruments as they can be grown perfectly without impurities and defects; (3) nano biology: including the genetic map of DNA and RNA; (4) nanomedicine: the invention, design, and production of nanoscale drugs.
-

For those of us living in Hong Kong, we may not have the opportunity to witness the aurora. However, for people residing in the far north or far south regions, the aurora is a mysterious dance in the night sky. The flickering pale green lights take on a shape of delicate strings that constantly transform, resembling gentle curtains swaying in the breeze, gracefully lingering in the serene and cold night sky. The intensity of the aurora varies, and at its brightest, it displays a dazzling array of colors, creating a captivating spectacle.
What is aurora?
Long ago, people believed that the aurora was simply sunlight reflecting off tiny ice particles in the sky. However, when scientists analyzed the spectrum of the aurora, they found that it didn't match the spectrum of sunlight, thus disproving this theory. On the other hand, the spectrum of the aurora shares similarities with the spectra produced by gases undergoing discharge at high voltages. In reality, the aurora is caused by the emission of gas molecules or atoms in the upper layers of Earth's atmosphere when they are struck by high-energy electrons from the Sun. In simple terms, when molecules or atoms are excited by electron collisions, they are raised to higher energy states or even ionized. When ions recapture electrons and return to their ground states, they emit light waves with specific wavelengths.
This model explains the colors of the aurora. Ultraviolet radiation from the Sun dissociates oxygen molecules into atoms, which become the primary constituents of the highest atmospheric layer, the ionosphere. When oxygen atoms are excited by electrons, they emit the predominant pale green light of the aurora. Electrons with higher energy can penetrate deeper into the atmosphere, exciting neutral nitrogen molecules that emit pink or magenta hues. Ionized nitrogen molecules emit purple-blue light. These secondary excitations enrich the colors of the aurora, adding delightful embellishments to the beautiful curtains in the sky.
The aurora is closely related to solar activity?
The high-energy electrons responsible for generating the aurora come from the Sun. The Sun is a blazing hot ball of gas, with temperatures exceeding millions of degrees in its outer atmosphere. In this atmosphere called the corona, atoms, primarily hydrogen, become ionized due to the high temperatures, forming a tenuous gas filled with free ions, mostly protons, and electrons. Eruptions from the solar corona continuously expel these ions and electrons into space, creating what is known as the solar wind. These charged particles, accompanied by the Sun's magnetic field, take approximately two days to reach Earth. Once the electrons encounter Earth's magnetic field, they become trapped and are guided toward the vicinity of the Earth's poles, where they collide with particles in the upper atmosphere, giving rise to the spectacular auroras.
Therefore, the occurrence of the aurora is closely linked to solar activity. The Sun follows an activity cycle known as the solar cycle, which has a duration of approximately eleven years. During these cycles, the Sun's activity reaches its peak. During these periods of solar storms, the Sun's surface may experience intense eruptions called solar flares, accompanied by the ejection of a large number of charged particles from the corona into space. These highly energetic particles can bring energy levels to thousands of times their usual levels, resulting in vibrant and heightened auroras that can even be visible as far as the United States.
-
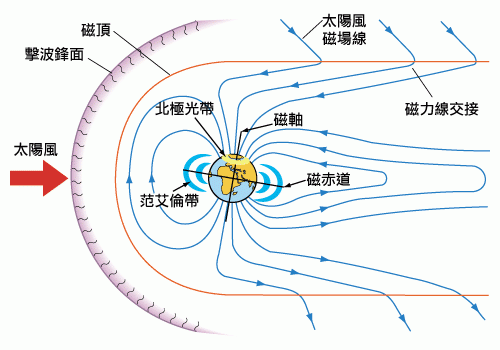
The interaction between the solar wind and Earth's magnetic field is a complex process.
Understanding the motion of charged particles when solar winds encounter the Earth's magnetic field is not simple. Figure two shows the interaction between the Earth's magnetic field and the solar wind, resulting in several regions. The Earth's magnetic field is molded into a cometary shape by the solar wind's magnetic field, and the magnetic field lines of both intersect at the boundary of the region, known as the magnetopause. When the solar wind passes through the magnetopause (Figure three), charged particles are influenced by the magnetic forces of the magnetic field. Protons are deflected to the right of Figure four, while electrons are deflected to the left (do you remember how to determine the direction of charged particle motion in a magnetic field using the right-hand rule?). The separation of these charges creates positive and negative electrodes and generates a current flowing from the positive electrode to the negative electrode. However, this current does not directly flow between the two electrodes; instead, it follows an interesting path: first, the current is influenced by the Earth's magnetic field and spirals along the magnetic field lines to the ionosphere, forming an elliptical conductive channel called the auroral zone. Finally, the current mainly exits from the other end of the ellipse and flows to the negative electrode. The aurora is formed within the auroral zone, a circular region centered around the North Pole, extending from northern latitudes to north-south, with a similar region near the South Pole. Therefore, auroras can only be seen in polar regions, either at the extreme north or south, and are difficult to observe near the equator.
-
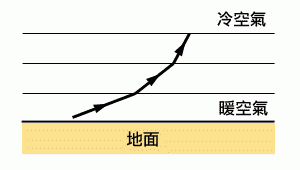
In the desert, a lost and starving person suddenly sees an oasis, only to realize upon approaching that it was just a mirage, a mere illusion. This is a common plot device in movies, but mirages do exist in reality. They are a result of nature playing tricks with light. The phenomenon of a mirage is caused by the refraction of light in the air, combined with total internal reflection.
What is Mirage? How is it formed?But what exactly is a mirage and how is it formed? To understand the formation of mirages, we must first understand why light is refracted in the air. Different temperatures of the air have different refractive indices, similar to different mediums. The air closer to the ground is warmer and has a lower refractive index. We can imagine the air as layers of different mediums, with each layer having a different refractive index. Closer to the ground, the refractive index is lower. Therefore, when light travels through the air, its path is affected by these different layers, as illustrated in the diagram.
This model also explains the colors of the aurora. Ultraviolet radiation from the Sun breaks down oxygen molecules into atoms, which become the primary component of the highest layer of the atmosphere called the ionosphere. When these oxygen atoms are excited, they emit the main pale green light of the aurora. High-energy electrons can penetrate into lower regions of the atmosphere and excite neutral nitrogen molecules, resulting in pink or reddish-pink glowing lights. Ionized nitrogen molecules emit a blue-purple light. These secondary excitations enrich the colors of the aurora, adding delightful fringes to this beautiful curtains.
On the other hand, it is important to understand what total internal reflection is. When light enters air at a slight angle from glass, part of the light is reflected back while the rest is refracted, exiting the glass. Since the refractive index of glass is higher than that of air, the refracted angle is always greater than the incident angle. As the incident angle becomes increasingly steep, the refracted light gets closer to the interface between the air and the glass until the incident angle exceeds the critical angle, where the light is entirely reflected back, without refracting. This phenomenon is known as total internal reflection.
The diagram below shows the path of light when a mirage occurs. Suppose there is an oasis, and the light emitted from point A is refracted by the air, taking a curved path. At point B, the light undergoes total internal reflection, causing it to travel upward. Subsequently, the light is refracted by the air again, and finally, it enters the observer's eye standing at point C, creating an illusion that the oasis is much closer to them. Humans discovered total internal reflection a long time ago and applied this phenomenon in various ways. For example, optical fibers, single-lens reflex cameras, and binoculars all utilize the principle of total internal reflection.
-

文: 張啟聰
-

Why can helicopters fly in the sky? Why is a vertical tail rotor necessary?
When you stand in front of a fan, you feel a cool breeze. If you increase the fan's size, the airflow becomes stronger, and the fan may even tilt slightly backward. You can imagine that a helicopter is like a very large fan, generating enough airflow to lift itself up! The rapidly rotating rotor blades act like the fan's blades, pushing the air downwards (action force). According to Newton's third law, the air provides an upward reaction force, causing the helicopter to ascend.
But what is the purpose of the vertical tail rotor? Let's first consider what happens without a tail rotor. Based on the principle of conservation of angular momentum, in the absence of external forces, the total angular momentum of the helicopter is zero. Assuming the rotor rotates clockwise, the body of the helicopter should rotate counterclockwise, perpetually spinning (Figure one). Therefore, a helicopter without a tail rotor cannot stay stable because it would continuously experience a counterclockwise torque. The rotating tail rotor provides a clockwise torque to the body, counteracting the torque generated by the rotor, and stabilizing the helicopter.
So, Bell's "Doraemon's Bamboo Copter" without a tail rotor would not be able to fly steadily; Doraemon would just keep spinning!
-
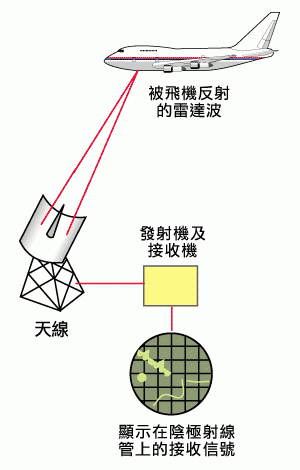
Stealth aircraft are not truly invisible, but rather they are designed to minimize their detectability by radar systems. To understand why they have this capability, let's consider how radar detection works. Radar detection involves the transmission of electromagnetic waves by an observer, which are then reflected back to the observer by the aircraft's metallic surfaces. By analyzing the time and direction differences between the transmitted and received waves, the observer can determine the position of the enemy aircraft.
Traditional aircraft have curved surfaces on their exteriors to reduce air resistance during flight. As a result, regardless of the angle at which electromagnetic waves hit the aircraft's surface, a portion of the waves is always reflected back to the original direction, resulting in a strong signal received by radar detectors. Modern stealth aircraft, on the other hand, have exteriors composed of numerous flat surfaces, making it difficult for electromagnetic waves to be reflected back to the original direction. Additionally, the materials used to construct the exteriors are carefully selected to effectively absorb the energy of radar signals. These materials often include carbon, carbon fiber composites, and magnetically absorbent ferrite materials. As a result, stealth aircraft appear nearly invisible in radar, resembling a small bird in the sky!

